Recommendations for the New COVID19 and RSV Immunizations
October 17, 2023
There are two new immunizations available for children this season, in addition to the traditional flu vaccine. Saugatuck Pediatrics will be ready to administer all three on opening (Flu, COVID19 and RSV-Beyfortus.) Here is more information to help you make an informed decision for your child(ren) about these recommended immunizations.
1. COVID 19 vaccine
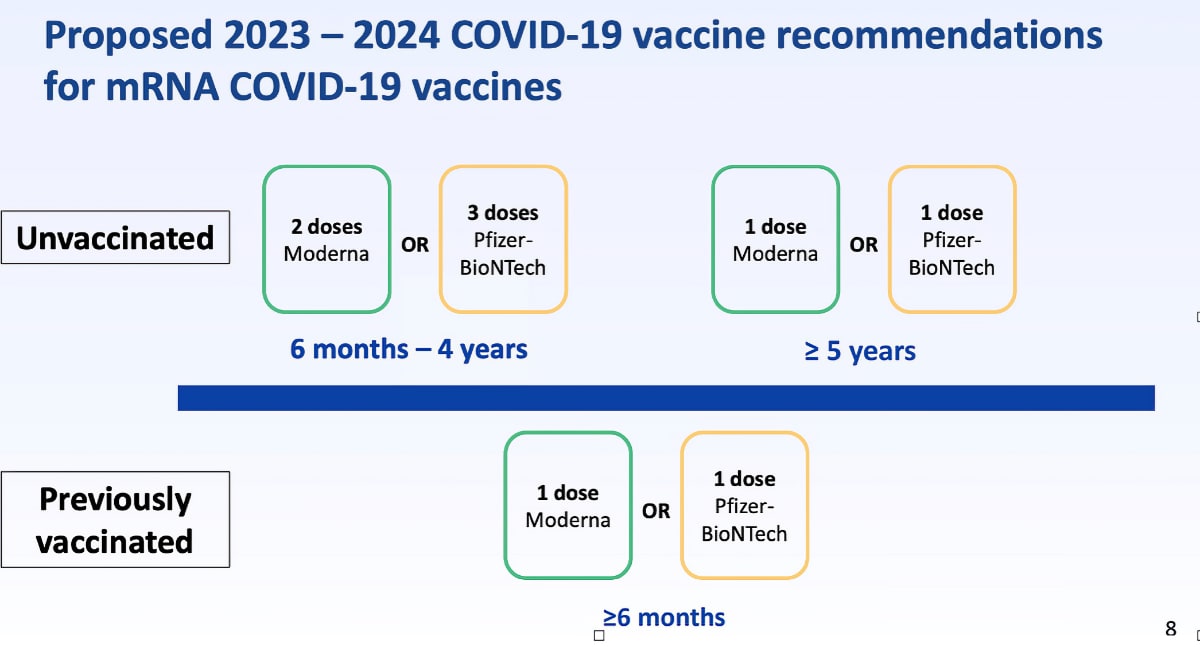
This year the COVID19 vaccine is a monovalent (single strain) vaccine targeting the current circulating variant XBB (an omicron variant.)
- All children aged 6 months and older are eligible for the vaccine.
- We will offer the Moderna version for children ages 6 mo to 18 years.
- If you started the COVID19 initial series at Village (or elsewhere), we can complete the series at Saugatuck IF THE VACCINE WAS MODERNA for ages 5 and under.
- For BOOSTER doses (after the primary series ages 5 and under, or anyone ages 6+) we can administer the Moderna brand (the CDC now allows mixing of vaccine type after the primary series is complete.)
- The incidence of myocarditis (heart inflammation), rarely seen in teens and young adults after the initial COVID19 vaccines, was significantly less (2 cases out of 650,000 vaccines) after the bivalent booster, likely due to the increased spacing between vaccines. This number is actually the same as the background risk of myocarditis (i.e. the risk of the condition without getting the vaccine.) The risk of myocarditis is much more common with natural COVID infections.
- If your child has recently had COVID you can wait up to 3 months to get the booster, but there is still a risk of waning immunity, and it is safe to get vaccinated as soon as COVID symptoms are gone and your child is no longer contagious (10 days from initial symptoms, with the first day of symptoms considered “day 0”.)
2. RSV (Nirsevimab or Beyfortus)
This is not a vaccine, but is still considered an “immunization.” It is an injection of long lasting antibodies against RSV that should provide protection throughout the RSV season (October thru end of March in Connecticut.) RSV, or Respiratory Syncytial Virus, is a cold virus that most of us get yearly, but infants are much more likely to suffer from severe symptoms, including wheezing, ear infections, and hospitalization (studies showed a 75% decrease in need to see an MD/hospitaization for RSV with this immunization, with antibodies lasting at least 150 days or more!) This immunization is approved for two groups:
- All infants aged newborn to 7 months born during or entering their first RSV season.
- Infants or children aged 8-19 months who are at increased risk for severe RSV disease and entering their second RSV season. This includes children with chronic lung disease, are severely immunocompromised or have cystic fibrosis with lung disease.
- This shot can be administered at the same time as other childhood vaccines, including COVID19.
- Maternal RSV vaccine was approved by the FDA on August 21, 2023. The CDC does not recommend nirsevimab for most infants born to a mother who received maternal RSV vaccine, except for infants where less than 14 days have elapsed between vaccination and birth.
AAP FAQs are available here.
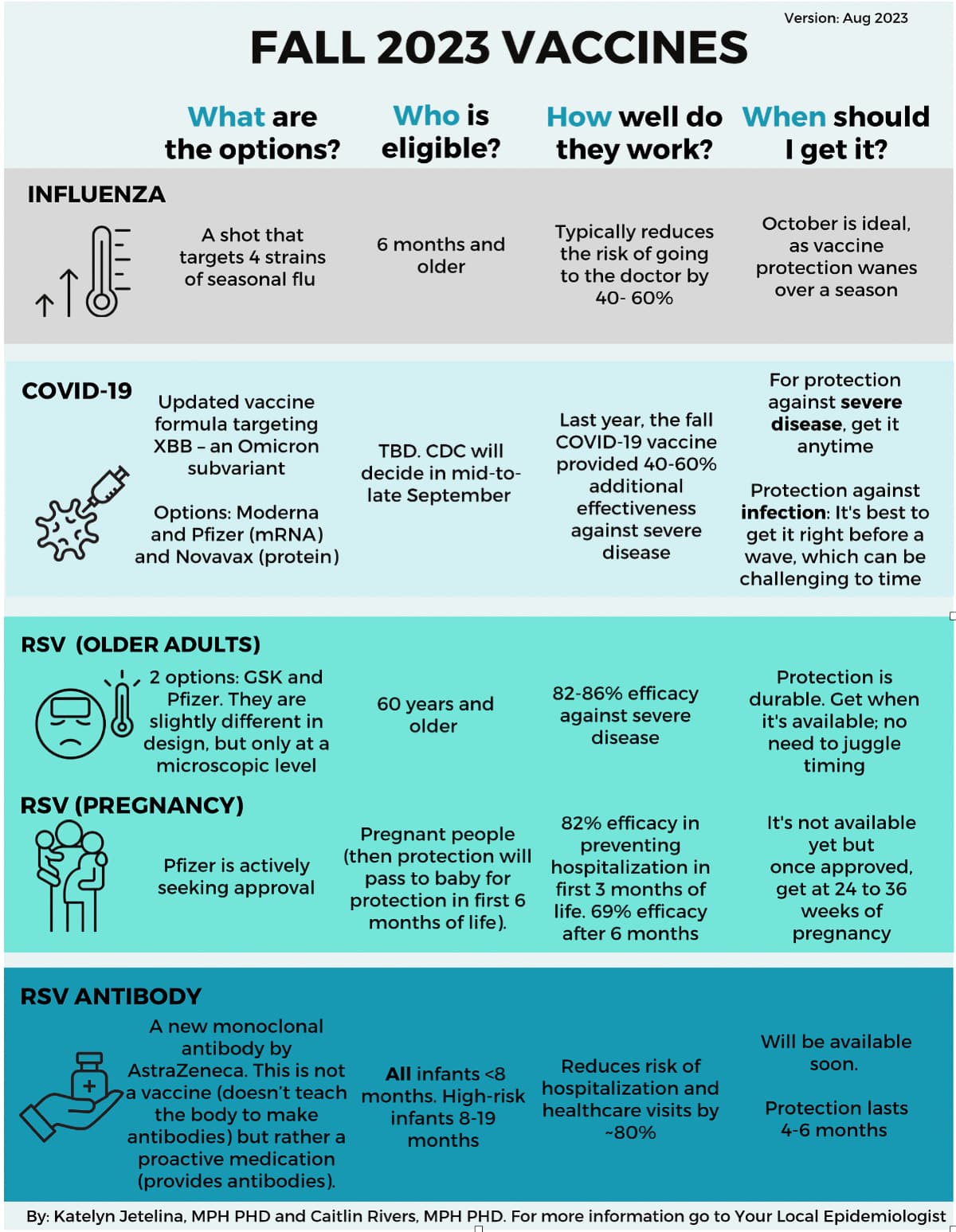
Here is a great chart from “Your Local Epidemiologist” summarizing this season’s immunization offerings: